Gates Foundation
Consulting, 09/2021—05/2022, Berkeley, CA
For fall 2021, I advised a Voyager Consulting team partnered with the integrated development division at the Bill and Melinda Gates Foundation. The foundation wanted to study substandard and falsified medicines (SFMs), a topic related to the foundation’s work in supporting pharmacuetical manufacturing. In low- and middle-income countries, nearly 1 in 10 medical products are substandard or falsified, resulting in over 1 million deaths and $21 billion in costs each year. The project sought to summarize existing literature on the scope of the problem and identify leading initiatives and technologies.
As preparation, I read Bottle of Lies by Katherine Eban, Phake by Roger Bate, and Countering the Problem of Falsified and Substandard Drugs by the Institute of Medicine. We split up the project into two phases: a secondary research phase to gather background knowledge and study prevalence, and a primary research phase with interviews of subject experts.
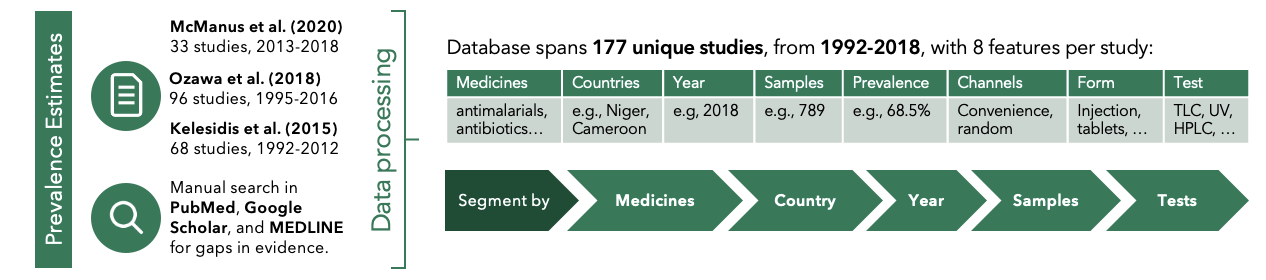
I oversaw the process of compiling an original database on SFM prevalence, regulatory strength, and country characteristics, covering more studies than any previous reviews. Afterwards, I helped consultant visualize and analyze the data to gain insights on the scope, causes, and impacts of SFMs. I streamlined a 55-page midpoint report and delegated follow-up deep dives.
In spring 2022, I took over as project leader and worked closely with the points of contact to significantly develop the research base of the project and write the ultimate report. We interviewed 11 subject experts and compiled over 200 studies into a 180-page report. I led the team to an in-person final presentation for the entire Integrated Development Team at the Gates Foundation. The report has gone on to inform Foundation strategy for investing in drug quality in sub-Saharan Africa.
